Coolstays supported 4 kelp survey sites along the Sussex coastline in 2023.
Coolstays chose to continue taking action towards protecting marine life in 2023, by sponsoring 4 kelp survey sites on the Sussex Kelp Recovery Project. For the second year running, Coolstays worked with GreenTheUK to fund innovative research led by Blue Marine Foundation and University of Sussex. This report outlines the findings from the 2023 survey report and includes footage from Coolstays survey sites.
Sites 14 to 17 were supported by Coolstays.
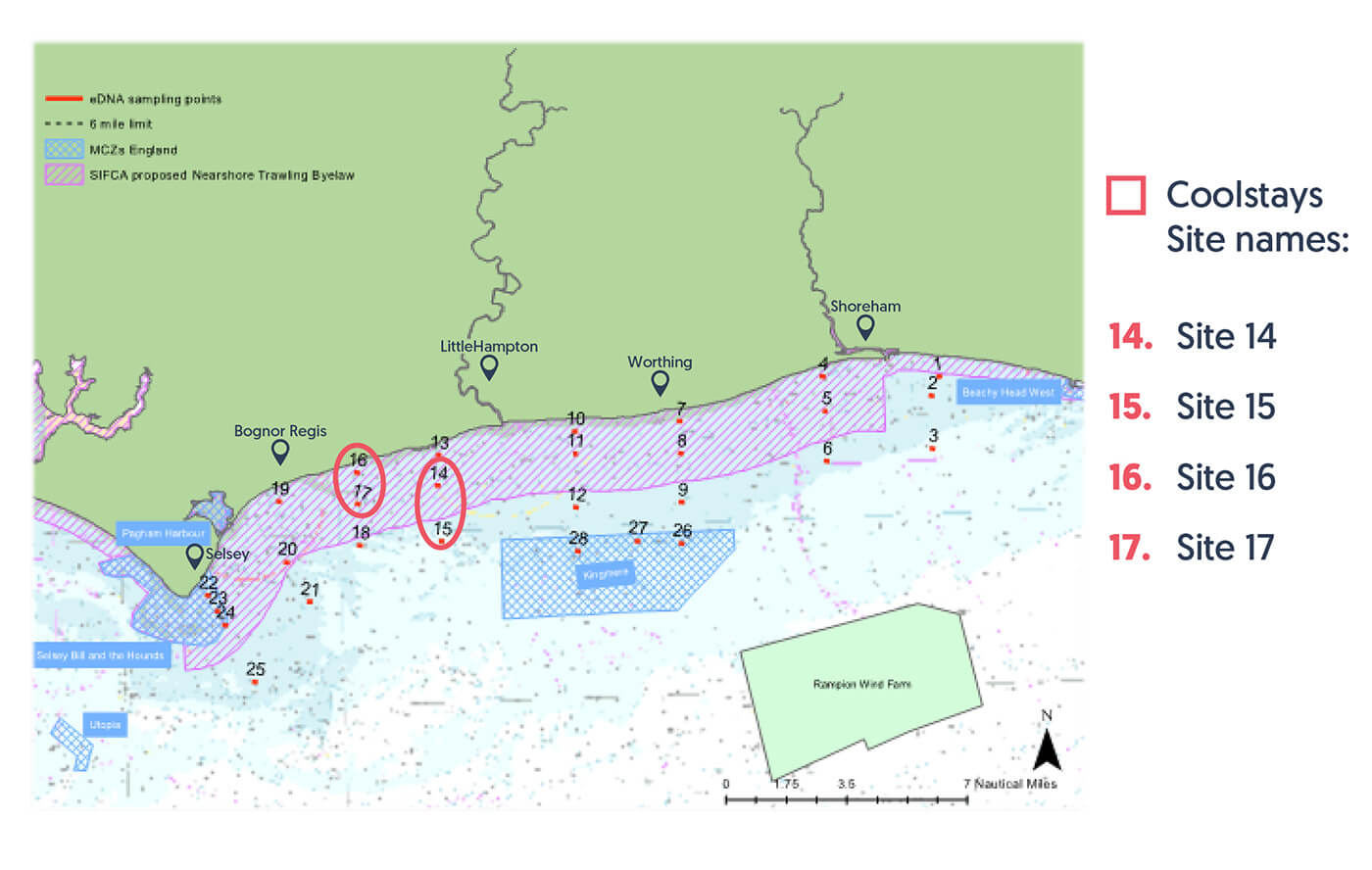
Coolstays supported 4 kelp survey sites along the Sussex coastline in 2023 to monitor the recovery of marine habitats and species following the Sussex Nearshore Trawling Byelaw using Baited Remote Underwater Video.

Summary
The waters off the Sussex coast historically supported dense kelp beds of mixed seaweed with at least six different species of kelps and other large brown macroalgae. However, since 1987, over 96 percent of the kelp beds in Sussex had disappeared. To help protect essential fish habitats and remove one of the key barriers to kelp recovery the Sussex IFCA Nearshore Trawling Byelaw was introduced in March 2021, excluding trawling from over 300 square kilometres of seabed. The Sussex Kelp Recovery Project (SKRP) was launched to support and enable the natural recovery of kelp and essential seabed habitats in the Nearshore Trawling Byelaw area.
One of the aims of the SKRP is to understand the ecological, social and economic value of kelp and the Sussex IFCA Nearshore Trawling Byelaw. This will allow benefits from the Byelaw and associated impacts to be evaluated and quantified. This programme is undertaken in collaboration with research organisations, regulators, fishermen, conservation groups, marine user groups and local communities.
Annual monitoring aims to record any changes in species diversity and composition following introduction of the Sussex Nearshore Trawling Byelaw and the anticipated recovery of kelp habitat. This will inform a better understanding of the trajectory of ecosystem recovery and the value to biodiversity of the Byelaw.
Methodology
In 2021, 2022 and 2023, the University of Sussex with support from Blue Marine deployed Baited Remote Underwater Videos (BRUVs) at 28 sites along the Sussex coast to assess diversity and abundance of mobile and benthic-associated species within and outside the Nearshore Trawling Byelaw area (Figure 1).
The BRUVs were equipped with GoPro HERO 8 cameras (Figure 2) to capture footage of the underwater fauna and flora at each site. Each BRUV was baited with one semi-thawed and one frozen scad (Trachurus trachurus), to attract animals.
The videos were subsequently reviewed to record the species observed (scientific name/common name), the time observed in the video, the number of individuals and the duration of observation.
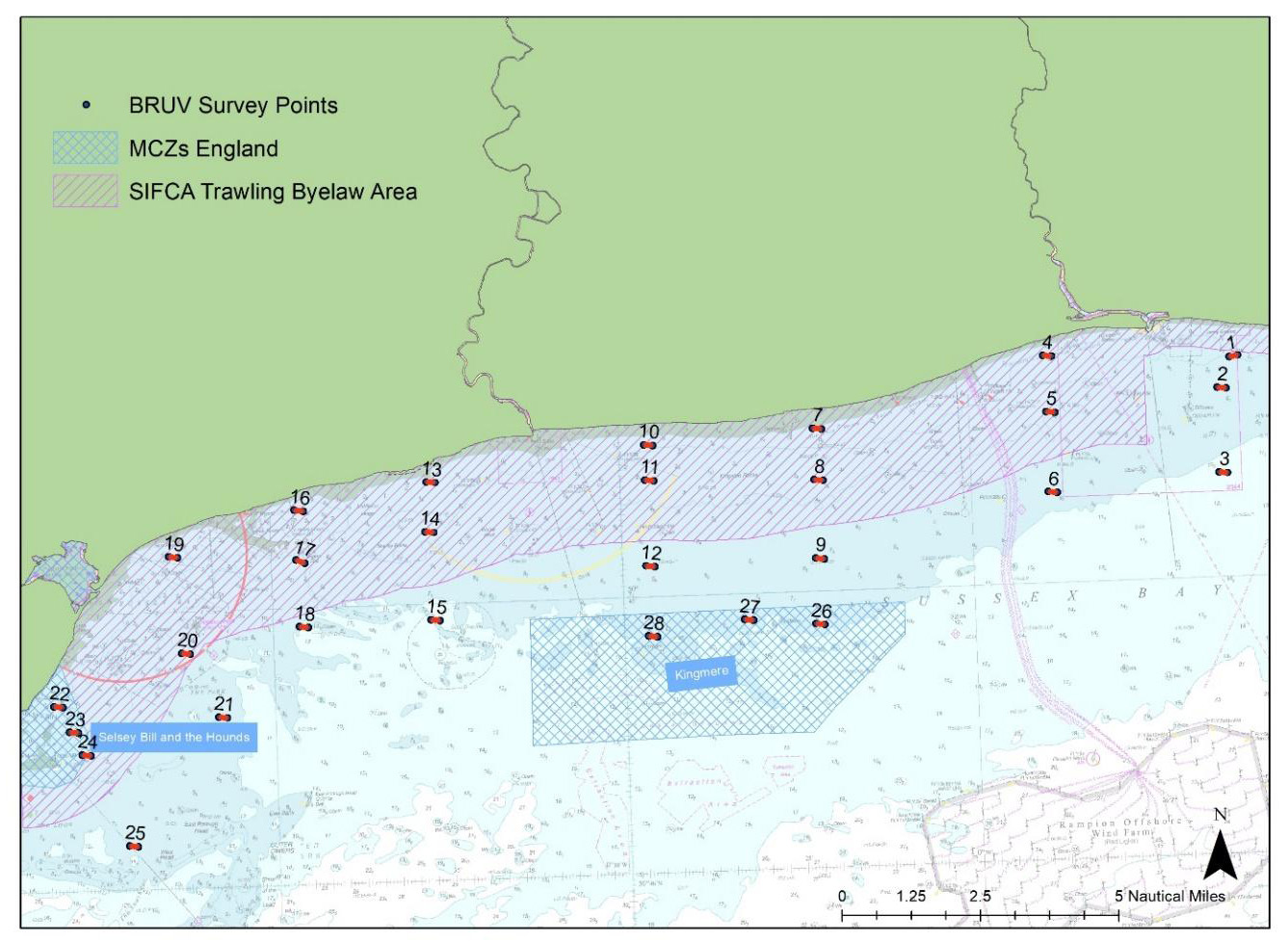 Figure 1: Map of the 28 sites along the Sussex Coast that were sampled with BRUVs in July 2021, 2022 and 2023. Note separate deployments of 3 BRUVs were undertaken at Swanage (50° 44.272' N, 0° 29.077' W) in 2021 and in Pullar Bank in 2022 and 2023 (50°40.530’N, 0°48.828’W) for reference against existing kelp dominated ecosystems.
Figure 1: Map of the 28 sites along the Sussex Coast that were sampled with BRUVs in July 2021, 2022 and 2023. Note separate deployments of 3 BRUVs were undertaken at Swanage (50° 44.272' N, 0° 29.077' W) in 2021 and in Pullar Bank in 2022 and 2023 (50°40.530’N, 0°48.828’W) for reference against existing kelp dominated ecosystems.
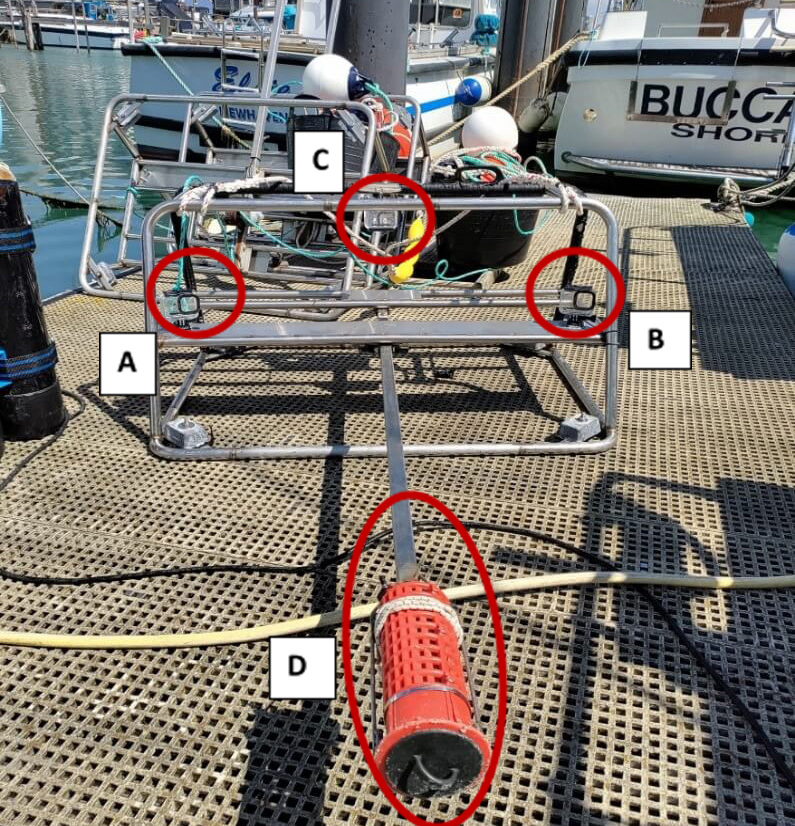 Figure 2: BRUV structure, including the two stereo GoPro Hero 8 cameras (A, B), the third GoPro Hero 8 camera set to time lapse for habitat (C) and the bait canister (D).
Figure 2: BRUV structure, including the two stereo GoPro Hero 8 cameras (A, B), the third GoPro Hero 8 camera set to time lapse for habitat (C) and the bait canister (D).
Preliminary Results for 2021-2023
- Overall, species diversity remained similar between the years of 2021, 2022 and 2023.
- A few species were identified in 2023 which had not been seen before, e.g. the Green Sea Urchin.
- No marked difference was seen inside vs outside the trawling exclusion zone, but Black Sea Bream and Conger Eels were more frequently seen inside the Byelaw zone and in Kingmere Marine Conservation Zone.
- As kelp and other essential fish habitats recover, the composition of mobile benthic species is likely to change and an increase in herbivores may be seen.
Appendices
Table A2: Species list, group and label
| Group | Scientific name | Sp_Label | Common name | Group | Scientific name | Sp_Label | Common name |
|---|---|---|---|---|---|---|---|
| Pelagic | Ammodytidae sp. | Ammo | Sandeel | Crustacea | Liocarcinus depurator | Lide | Harbour Crab |
| Echinoderm | Amphipholis squamata | Amsq | Brittle Star | Demersal | Lipophrys pholis | Liph | Shanny |
| Mollusc | Aplysia puncata | Appu | Sea Hare | Crustacea | Macropodia rostrata | Maro | Long-legged Spider Crab |
| Mollusc | Arctica islandica | Aris | Icelandic Cyprine | Crustacea | Maja brachydactyla | Mabr | Spiny Spider Crab |
| Echinoderm | Asterias rubens | Asru | Common Starfish | Demersal | Mullus surmuletus | Musu | Red Mullet |
| Mollusc | Buccinum undatum | Buun | Common Whelk | Pelagic | Mustelus asterias | Muas | Starry Smooth Hound |
| Crustacea | Cancer pagurus | Capa | Edible Crab | Crustacea | Necora puber | Nepu | Velvet Swimming Crab |
| Mollusc | Calliostoma zizyphinum | Cazi | Painted Topshell | Mollusc | Nucella labillus | Nula | Dog Whelk |
| Crustacea | Carcinus maenas | Cama | Shore Crab | Crustacea | Pagarus bernardus | Pabe | Common Hermit Crab |
| Demersal | Centrolabrus exoletus | Ceex | Rock Cook | Crustacea | Palaemon serratus | Pase | Common Prawn |
| Demersal | Chelidonichthys lucerna | Chlu | Tub Gurnard | Demersal | Pollachius pollachius | Popo | Pollack |
| Demersal | Chirolophis ascanii | Chas | Yarrells Blenny | Demersal | Pomatoschistus microps | Pomi | Common Goby |
| Pelagic | Conger conger | Coco | Conger Eel | Demersal | Pomatoschistus minutus | Pomi | Sand Goby |
| Crustacea | Corystes cassivelaunus | Coca | Masked Crab | Demersal | Pomatoschistus pictus | Popi | Painted Goby |
| Demersal | Ctenolabrus rupestris | Ctru | Goldsinny | Demersal | Raja clavata | Racl | Thornback Ray |
| Demersal | Dasyatis pastinaca | Dapa | Common Stingray | Demersal | Raja undulata | Raun | Undulate Ray |
| Demersal | Dicentrarchus labrax | Dila | Bass | Pelagic | Scomber scomber | Scsc | Mackerel |
| Crustacea | Diogenes pugilator | Dipu | Hermit Crab | Pelagic | Scyliorhinus canicula | Scca | Small Spotted Catshark |
| Mollusc | Doris pseudoargus | Dops | Sea Lemon | Mollusc | Sepia officinalis | Seof | Common Cuttlefish |
| Demersal | Eutrigla gurnadus | Eugu | Grey Gurnard | Demersal | Sparus aurata | Spau | Gilthead Sea Bream |
| Crustacea | Galathea spp. | Gasp | Squat Lobster | Demersal | Spondyliosoma cantharus | Spca | Black Seabream |
| Pelagic | Galeorhinus galeus | Gaga | Tope Shark | Mollusc | Steromphala cineraria | Stci | Grey Topshell |
| Mollusc | Glycymeris glycymeris | Glgl | Dog Cockle | Demersal | Symphodus melops | Syme | Corkwing Wrasse |
| Demersal | Gobiusculus flavescens | Gofl | Two-spot Goby | Demersal | Thorogobius ephippiatus | Thep | Leopard-Spotted Goby |
| Demersal | Gobuis paganellus | Gopa | Rock Goby | Demersal | Trisopterus luscus | Trlu | Bib |
| Crustacea | Goneplax rhomboides | Gorh | Angular Crab | Demersal | Trisopterus minutus | Trmi | Poor Cod |
| Crustacea | Hyas araneus | Hyar | Great Spider Crab | Mollusc | Tritia reticulata | Trre | Netted Dog Whelk |
| Crustacea | Inachus dorsettensis | Indo | Scorpion Spider Crab | Mollusc | Trivia monacha | Trmo | European Cowries |
| Demersal | Labrus bergylta | Labe | Ballan Wrasse | Mollusc | Trochidae | Trtr | Topshells |
| Demersal | Labrus mixtus | Lami | Cuckoo Wrasse | - | - | - | - |
Acknowledgements
Thank you once again to Coolstays for their support of the Sussex coastline and contributing towards this important research on an essential marine habitat for biodiversity.
Thanks to PhD student Alice Clark, Professor Mika Peck, Dr Valentina Scarponi and Masters students at University of Sussex for undertaking surveys, data analysis and collation of images and footage. Thanks also to Neville Blake, skipper of the New Dawn charter boat.
Thanks to all GreenTheUK sponsors for support of this research programme.
This case study was adapted from a report published in December 2023: A collaborative study between Blue Marine Foundation and University of Sussex as part of the Sussex Kelp Recovery Project. The report authors were Sam Fanshawe of Blue Marine Foundation and Alice Clark, Mika Peck and Valentina Scarponi from the University of Sussex, School of Life Sciences, Ecology and Evolution.
If you have any questions about the research, please contact Alice Clark (University of Sussex): ac831@sussex.ac.uk
UN's Sustainable Development Goals
As a GreenTheUK partner, you support projects that are in line with the UN Sustainable Development Goals.

Take urgent action to combat climate change and its impacts.

Conserve and sustainably use the oceans, seas and marine resources for sustainable development.