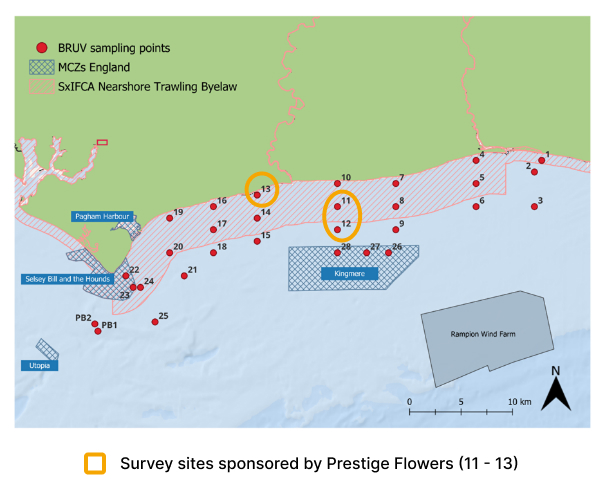
Prestige Flowers supported 3 kelp survey sites along the Sussex coastline in 2024
Prestige Flowers is dedicated to protecting marine biodiversity and took action in 2024 by sponsoring 3 kelp survey sites as part of the Sussex Kelp Recovery Project, in collaboration with Blue Marine Foundation and GreenTheUK. Through this partnership, Prestige Flowers helped fund vital research led by Blue Marine Foundation and the University of Sussex, enhancing our understanding of species abundance, richness, and the ecological impact of the Sussex Nearshore Trawling Byelaw supporting kelp recovery.
This report reveals key insights from the 2024 survey, showcasing progress made—along with exclusive footage from Prestige Flowers’s survey sites, using Baited Remote Underwater Video (BRUV).
Sites 11 to 13 were supported by Prestige Flowers.
Summary
The waters off the Sussex coast historically supported dense kelp beds with at least six different species of kelp and other large brown macroalgae. However, since 1987, over 96 percent of the kelp beds in Sussex have disappeared. Years of intensive bottom trawling and other human pressures, such as poor water quality, sedimentation and severe storm events, has decimated this valuable marine habitat that once stretched along more than 40 km of coastline, from Selsey to Shoreham.
To help protect essential fish habitats and remove one of the key barriers to kelp recovery, the Sussex Inshore Fisheries and Conservation Authority (IFCA) Nearshore Trawling Byelaw was introduced in March 2021. The Byelaw excludes trawling from over 300 square kilometres of seabed, removing a key pressure from the area and giving kelp a chance to recover. The Sussex Kelp Recovery Project (SKRP) was launched to support and enable the natural recovery of kelp and essential fish habitats in the Nearshore Trawling Byelaw area.
One of the aims of the SKRP is to understand the ecological, social and economic value of kelp and the Sussex IFCA Nearshore Trawling Byelaw. This will allow benefits from the Byelaw and associated impacts to be evaluated and quantified. This programme is undertaken in collaboration with research organisations, regulators, fishermen, conservation groups, marine user groups and local communities.
Blue Marine, as a member of the SKRP, has secured funding to support a number of projects in 2021, 2022, 2023 and 2024 to collect ecological, fisheries and socio-economic baseline data within the Byelaw area. One of these projects aims to assess, over time, the effects of the Sussex IFCA Nearshore Trawling Byelaw and other spatial management areas on mobile (nekton and benthic-associated) species.
Annual monitoring aims to record any changes in species diversity and composition following introduction of the Byelaw and the anticipated recovery of kelp habitat. This will inform a better understanding of the trajectory of ecosystem recovery and the value to biodiversity of the Nearshore Trawling Byelaw.
In 2021, 2022, 2023 and 2024, the University of Sussex, with support from Blue Marine Foundation, deployed Baited Remote Underwater Videos at 30 sites along the Sussex coast to assess diversity and abundance of mobile and benthic-associated species within and outside the Nearshore Trawling Byelaw area. Prestige Flowers sponsored 3 kelp sites in 2024, in partnership with GreenTheUK and Blue Marine Foundation.
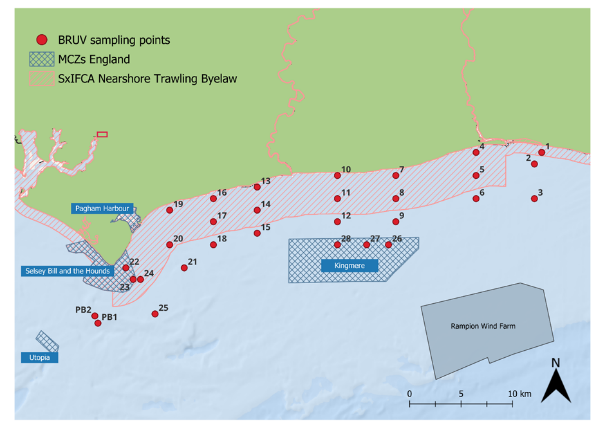 Figure 1: Map of the 28 sites along the Sussex Coast that were sampled with BRUVs in July 2021, 2022, 2023 and 2024. Note separate deployments of 3 BRUVs were undertaken at Swanage (50° 44.272' N, 0° 29.077' W) in 2021 and at Pullar Bank (PB1 and PB2) in 2022-2024 for reference against existing kelp dominated ecosystems.
Figure 1: Map of the 28 sites along the Sussex Coast that were sampled with BRUVs in July 2021, 2022, 2023 and 2024. Note separate deployments of 3 BRUVs were undertaken at Swanage (50° 44.272' N, 0° 29.077' W) in 2021 and at Pullar Bank (PB1 and PB2) in 2022-2024 for reference against existing kelp dominated ecosystems.
Key Findings
- A total of 86 different species have been identified across 4 years.
- A total of 54 species were identified in 2021 (27 vertebrate and 27 invertebrate species).
- A total of 43 species were identified in 2022 (24 vertebrate and 19 invertebrate species).
- A total of 62 species were identified in 2023 (32 vertebrate and 30 invertebrate species).
- A total of 57 species were identified in 2024 (29 vertebrate and 28 invertebrate species).
- In 2024, 4 species were identified that had not been seen in previous years, most were only seen once or twice.
- There is evidence of an increase in pelagic-neritic species (e.g. Atlantic mackerel, sand eels, mullets) since 2021.
- Detection of black seabream and two-spot goby were more highly linked to sites inside the Byelaw area and in MCZ-Kingmere; small spotted catshark were linked to sites outside the Byelaw area and in MCZ-Selsey Bill and the Hounds; rock cook, pollock and ballan wrasse were linked to kelp-control sites at Pullar Bank.
- The species richness between the four years of sampling was found to be significantly different. Species richness was significantly higher in 2023 than 2022, potentially linked to high algal cover present in 2022 - which obscures benthic-associated invertebrates on the videos.
- The evenness of the communities is relatively low every year for both invertebrates and vertebrates, suggesting that there are a few common species in high abundance and a lot of species with low abundances.


Methodology
Study Area
Surveys were carried out at 28 different sites between Selsey Bill and Shoreham-by-Sea, inside and outside the Byelaw area (Figure 1). The sites were chosen to match the towed video transects deployed by Sussex Inshore Fisheries and Conservation Authority, to complement their habitat data (Mallinson & Yesson, 2020). Two additional sites were sampled in Swanage in 2021 and in Pullar Bank from 2022 to 2024, to provide further comparative information with existing kelp dominated ecosystems. Samples were collected between the 5th and the 21st of July 2021, the 11th and 27th of July 2022, the 10th of July and the 11th of September 2023 and the 8th and the 30th of July 2024. The survey period in 2023 extended into September due to poor weather conditions in late July and August of that year.
There are five treatments:
- Nearshore Trawling Byelaw (“Inside”) - areas inside the designated Byelaw where bottom trawling is banned and where previous dense kelp beds were present;
- MCZ-K - Kingmere Marine Conservation Zone;
- MCZ-S - Selsey Bill and the Hounds Conservation Zones;
- Open Control (“Outside”) – areas outside the Byelaw area and MCZs; and
- Kelp control – areas with existing healthy kelp ecosystems
Baited Remote Underwater Video (BRUV)
The methodology employed was based on one developed by Plymouth University and used as part of the Lyme Bay MPA (Marine Protected Area) monitoring (Sheehan et al. 2013), to obtain quantitative data on mobile organisms in different experimental treatments.
At each site, three different BRUVs (Figure 2) were deployed 150 m from one another, for 65-70 min before being retrieved.
BRUVs were equipped with 3 GoPro HERO 8 cameras. The settings of the two stereo GoPros facing the bait canister were standardised to be: 1080p, linear and 30 FPS. The third camera was placed to face the back of the rig and set to record a time-lapse series of pictures of the surrounding habitat. The bait canister of each BRUV rig was filled with one semi-thawed and one frozen scad (Trachurus trachurus), which were sliced into 4 different pieces to enhance the strength of their scent.
For each BRUV deployment the following measurements were taken; GPS (Latitude and Longitude), date, time of deployment and depth (based on sonar).
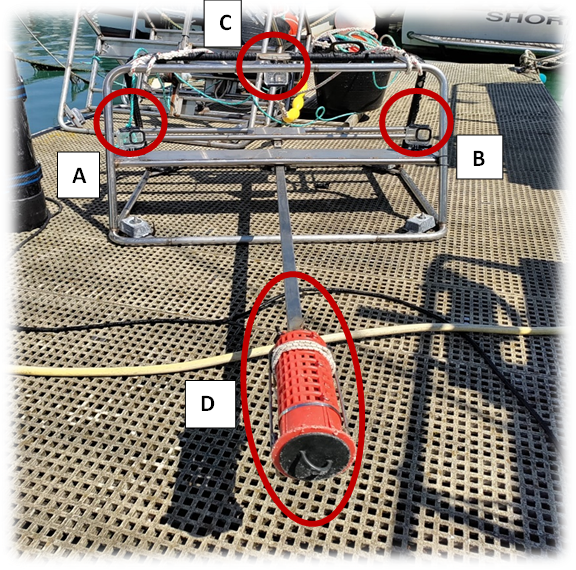 Figure 2: BRUV structure, including the two stereo GoPro Hero 8 cameras (A, B), the third GoPro Hero 8 camera set to time lapse for habitat (C) and the bait canister (D).
Figure 2: BRUV structure, including the two stereo GoPro Hero 8 cameras (A, B), the third GoPro Hero 8 camera set to time lapse for habitat (C) and the bait canister (D).
The videos were subsequently reviewed to record the species observed (scientific name/common name), the time observed in the video, the number of individuals and the duration of observation.
The biotype at each site was categorised based on the BRUV footage into categories: gravel-cobbles, mixed sediment, sand, cobbles and pebbles and rocky reef.
The percentage of macroalgal cover was also recorded for each site (0% = 0, 1-20% = 1, 20-40% = 2, 40-60% = 3, 60-80% = 4, 80-100% = 5, 6 = kelp). Data for water temperature and tidal coefficient was also collected.
The maximum number of individuals on screen (MaxN) for each species recorded at each site was used in data analysis. MaxN is considered a conservative estimate of relative abundance, eliminating the chance of counting the same individual multiple times.
Statistical analyses explored Abundance, Species Richness and Effective Number of Species (ENS) between treatment areas (Jost, 2007). Multivariate analyses (CCA) were carried out to assess and visualise the relationship between the community composition and the environmental variables (macroalgal cover, tidal coefficient, depth, treatment, biotype). All analyses were performed using R statistical programme and R Studio with the vegan package.
Vertebrate species were grouped by niche type (benthopelagic, demersal, pelagic-neritic and reef-associated) using the R package Rfishbase. Invertebrate species were grouped by order: Crustacea, Mollusc and Echinoderm.
Preliminary Results for 2021, 2022, 2023 and 2024
Species Diversity
Over the last 4 years, a total of 86 different species (42 vertebrates and 46 invertebrates) have been identified across our 30 Sussex sites. In 2024, 57 different species were noted on the BRUVs, 28 of these were invertebrates and 29 of these were vertebrates, compared to 54 species in 2021, 43 in 2022 and 62 in 2023.
Overall, a significant difference in species richness was observed between sampling years. Species richness was significantly higher in 2023 than 2022, potentially linked to high algal cover present in 2022 - which obscures benthic-associated invertebrates on the videos.
Further, the model highlighted the importance of biotype, showing a significant positive relationship with the “Rocky-reef” biotype, where species richness was higher, while “sandy” sites exhibited a negative relationship with species richness. Depth was also a significant factor in the model, so species richness increased as depth increased.
Although water temperature did not directly affect species richness, there was a significant increase in water temperature between 2021 and subsequent sampling years.
Across the four years of sampling, 26 species were consistently identified each year. Four new species were detected in 2024 that had not been detected in previous years (Figure 3; Table 1).
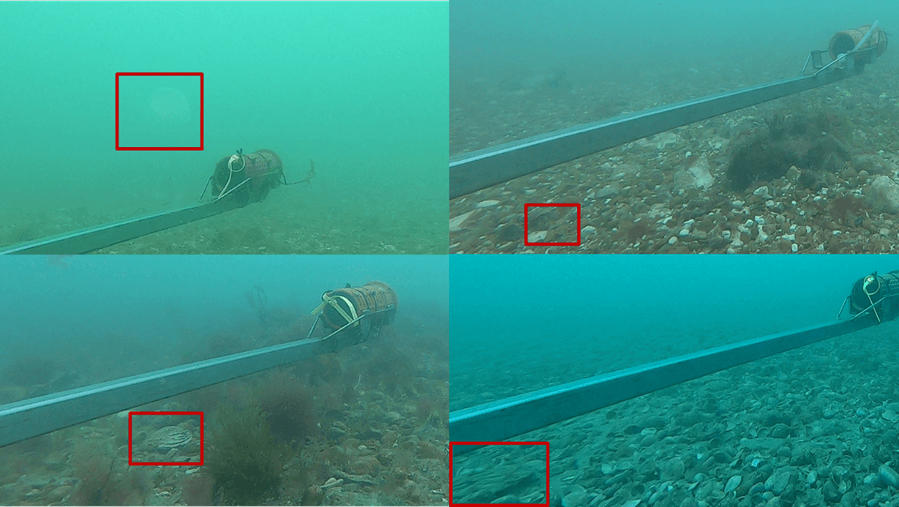 Figure 3: BRUV images of species identified in 2024 but not previous years: A) moon jelly (Aurelia aurita), B) reticulated dragonet (Callionymus reticulatus), C) pacific oyster (Crassostrea gigas), and D) king scallop (Pecten maximus).
Figure 3: BRUV images of species identified in 2024 but not previous years: A) moon jelly (Aurelia aurita), B) reticulated dragonet (Callionymus reticulatus), C) pacific oyster (Crassostrea gigas), and D) king scallop (Pecten maximus).
Table 1: Species unique to specific years
| 2021 | 2022 | 2023 | 2024 | |
|---|---|---|---|---|
| Unique Species |
|
|
|
|
Abundance
The species identified were classified by order for invertebrates (crustacea, echinoderm, mollusc) and by niche for the vertebrates (benthopelagic, demersal, pelagic-neritic, reef-associated).
Changes over the four years in species abundance within each group was tested (Figure 4). Overall, between 2021 and 2024, there was a significant increase in pelagic-neritic species, while no significant changes were noted for other species groups. However, there were some changes in abundance between other years. For example, between 2021 and 2022, there was a decrease in crustacea, echinoderms, molluscs and demersal species, and between 2021 and 2023, there was a significant decrease in echinoderms, molluscs, demersal and reef-associated species. Pelagic-neritic species also increased in 2023.
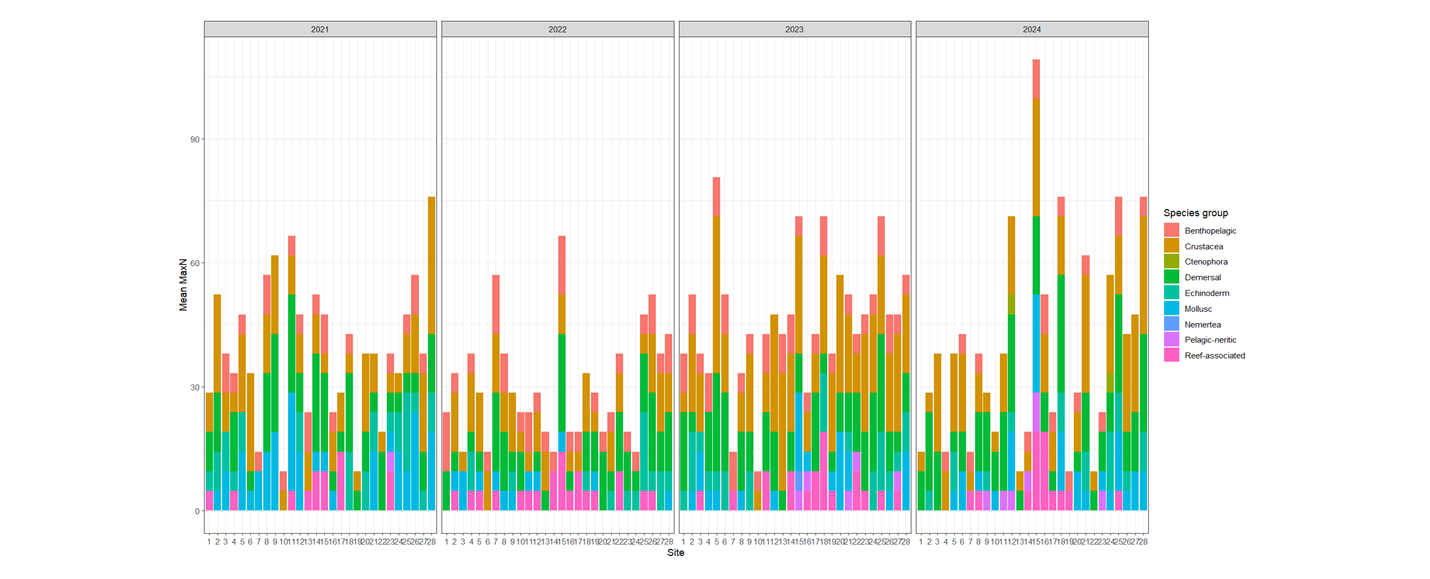 Figure 4: Stacked barplot showing the average (mean) MaxN of each species group across the three years of sampling for 28 Sussex sites, providing overview of community composition per site.
Figure 4: Stacked barplot showing the average (mean) MaxN of each species group across the three years of sampling for 28 Sussex sites, providing overview of community composition per site.
Rank-abundance curves were plotted to gain an understanding of the most commonly detected species each year and to explore the change in evenness of the vertebrate and invertebrate communities each year.
Overall the most commonly detected species in each year were as follows:
- 2024: Altantic mackerel (Scomber scombrus), common brittlestarts (Ophiothrix fragilis) and bib (Trisopterus luscus).
- 2023: Altantic mackerel (Scomber scombrus), Black seabream (Spondyliosoma cantharus) and common brittlestars (Ophiothrix fragilis).
- 2022: black seabream (Spondyliosoma cantharus), common brittlestarts (Ophiothrix fragilis) and bib (Trisopterus luscus).
- 2021: common brittlestars (Ophiothrix fragilis), European coweries (Trivia monacha) and topshells (Trochidae spp.).
The evenness of the communities is relatively low every year for both invertebrates and vertebrates, suggesting that there are a few common species in high abundance and a lot of species with low abundances.
Community Composition
A separate CCA was performed to include the kelp-control sites to see how the Sussex sites compared to these.
The plot shows the different sites coloured by treatment type (Figure 5). The sites from the kelp-control treatment are also separated from the other sites which highlights the difference in community structure of those sites to the others.
Rock cook, pollock and ballan wrasse were linked to the “kelp-control” sites. Black seabream and two-spot goby were linked to the “inside” sites and “MCZ-Kingmere”. Conger eels were associated with both the “inside” and “outside” sites and small-spotted catshark were linked to “outside” sites and “MCZ-Selsey Bill and the Hounds”.
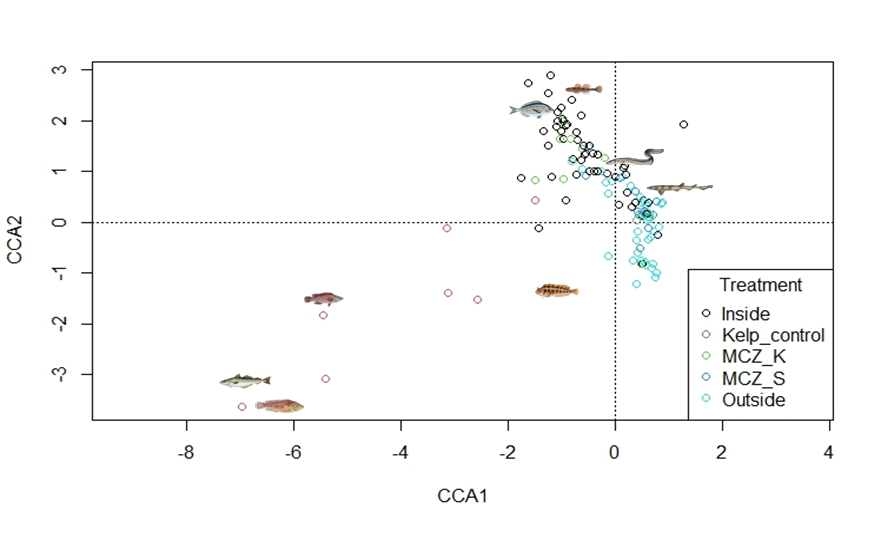 Figure 5: CCA of Sussex and kelp-control sites over 4 years with sites coloured by treatment type. Common species found within each species have been added to the plot.
Figure 5: CCA of Sussex and kelp-control sites over 4 years with sites coloured by treatment type. Common species found within each species have been added to the plot.
Discussion
Since the implementation of the Nearshore Trawling Byelaw, relatively little change in Abundance, Species Richness and Effective Number of Species has been found within the study area. However, a significant increase in pelagic-neritic species, predominantly Atlantic mackerel, has been seen since 2021. This could be an indication of positive change as an increase in these shoaling species will bring in more apex predators into the ecosystem, such as bluefin tuna and dolphins, which are crucial to a healthy, balanced ecosystem (Baum & Worm, 2009; Mariani et al., 2017).
As the Byelaw was introduced in March 2021, and these surveys were only conducted over the 4.5 years since designation, it is not unexpected to see little change in this timeframe. Although some marine taxa have seen recovery in under five years, the full recovery of ecosystems, which have endured decades of degradation, more commonly take a minimum of 15-25 years (Borja et al., 2010). It is possible that some ecosystems may never fully recover to their original historical state, but rather end up in an alternate state (Belzunce et al., 2001; Hering et al., 2010). This could potentially happen in Sussex, due to the impact of other environmental factors such as increased sedimentation and water temperature since the late 80s, when dense kelp beds were present in the area. However, our results do suggest that the community structure of the biodiversity present in Sussex has changed since 2021 and that this is driven partly by macroalgal cover. However, the duration of the annual BRUV surveys has not yet been sufficient to determine whether these changes are part of natural cyclical changes in the ecosystem or indicative of ecosystem recovery. Consequently, continued monitoring over a longer period is recommended.
Our results suggest that an increase in macroalgal cover correlates with a reduction in species richness. This is a surprising result as macroalgae is known to benefit many organisms, acting as nursery grounds for fish species and a valuable food source for both invertebrates and vertebrate species (Norderhaug, Fredriksen & Nygaard, 2003). However, this is likely due to increased macroalgae cover affecting the visibility on the BRUV footage, leading to false negatives. The macroalgal cover in 2021, 2023 and 2024 was lower than in 2022 which may explain why the species richness in those three years is very similar (Figure A3). Poor visibility is one of the limitations of using BRUV systems as a biomonitoring method (Unsworth, 2014). To address this limitation, we complement our BRUV surveys with additional biomonitoring tools such as environmental DNA (not discussed in this report).
Several species were identified in 2024 that had not been detected in previous years. This could be a sign of the beginning of an ecosystem shift. However, as the majority of these species were seen in low abundance, further research will be needed to corroborate these findings. There are several other reasons which could explain why these species have not been seen in previous years. Some species may be more cryptic or rare and were potentially only detected by chance in 2024. These species may have simply not been in the area at the time of the BRUV deployments in previous years. The likelihood of misidentification is minimal, thanks to the meticulous identification process conducted by students utilising the "The Diver’s Guide to Marine Life of Britain and Ireland" identification book, which is subsequently cross-verified by staff members.
Our results have also highlighted the most common species seen on the BRUVs each year. These have changed slightly over the last few years of sampling. Notably, there was a large increase in black seabream (S. cantharus) between 2021 and 2023. Black seabream is an important commercial species for local fisheries (Gonzála Pajuelo J M & Nespereira, 2004). Therefore, observing an increase in their abundance suggests that removing trawling pressure is positively affecting this species, likely by safeguarding their habitats and nests from damage caused by trawling, and by reducing the frequency at which they are targeted. However, in 2024, very few juvenile black seabream were seen and the numbers were similar to 2021. A recent study by Davies et al. (2024) showed that black seabream display interannual fidelity to spawning sites in Kingmere MCZ. Therefore, this sudden drop in numbers on the BRUV surveys is surprising and cannot be explained by the environmental variables collected, but may be due to natural population fluctuations.
The analysis investigating the evenness of the communities over the three sampling years also revealed that there are a few common species in high abundance and many species in low abundance. This relationship is known as the distribution-abundance relationship and is commonly seen in most natural assemblages (Preston, 1948). It is important to note that the biases inherent to BRUV sampling, such as the overrepresentation of certain species associated to bait choice such as scavenger species, and a tendency to favour less cryptic species (Harvey 2007, Coghlan 2017, Jones 2020). Although the species detected most frequently and in highest abundance are likely to be present in higher numbers than others in the ecosystem, it is also possible that these are the species which are most attracted to the bait used for these surveys.
Our multivariate analyses revealed that macroalgal cover and treatment type affect community composition. As it is known that certain species will be more associated with different levels of algal cover, these results are not surprising. However, as discussed above, increased algal cover will reduce visibility on the BRUV surveys, resulting in certain species being missed. The change in community composition between the different treatment types suggests that different zones of protection may have an effect on community structure. Although it must be noted that areas outside the NTB are also in deeper waters, therefore depth will also have an effect on the species present. Our kelp control sites showed a different community composition, with species such as ballan wrasse, rock cook and pollack being strongly associated with them. It is expected that if macroalgae, and especially kelp – which plays a major role in generating habitat heterogeneity - returns to the rest of Sussex, the other Sussex sites will begin to more closely resemble those from Swanage and Pullar Bank.
Conclusion
From the results of the BRUV surveys over the period 2021 to 2024, a significant change has been seen in species richness but not in ENS in the study area since the introduction of the Nearshore Trawling Byelaw. We are beginning to see a slight shift in species composition over the years linked to the abundance of macroalgae and the treatment in which the sites are found. More in-depth studies will be carried out in the following months by Sussex undergraduate students, Masters students and PhD students to further explore this data and get a better understanding of the species diversity and community composition of the Sussex ecosystem over the last three years.
Appendices
Table A1: Species list, group and label
| Group | Scientific name | Sp_Label | Common name | Group | Scientific name | Sp_Label | Common name |
|---|---|---|---|---|---|---|---|
| Pelagic | Ammodytidae sp. | Ammo | Sandeel | Demersal | Labrus mixtus | Lami | Cuckoo Wrasse |
| Echinoderm | Amphipholis squamata | Amsq | Brittle Star | Crustacea | Liocarcinus depurator | Lide | Harbour Crab |
| Mollusc | Aplysia punctata | Appu | Sea Hare | Demersal | Lipophrys pholis | Liph | Shanny |
| Mollusc | Arctica islandica | Aris | Icelandic Cyprine | Crustacea | Macropodia rostrata | Maro | Long-legged Spider Crab |
| Echinoderm | Asterias rubens | Asru | Common Starfish | Crustacea | Maja brachydactyla | Mabr | Spiny Spider Crab |
| Semaeostomeae | Aurelia aurita | Auau | Moon Jellyfish | Demersal | Mullus surmuletus | Musu | Red Mullet |
| Mollusc | Buccinum undatum | Buun | Common Whelk | Pelagic | Mustelus asterias | Muas | Starry Smooth Hound |
| Crustacea | Cancer pagurus | Capa | Edible Crab | Crustacea | Necora puber | Nepu | Velvet Swimming Crab |
| Demersal | Callionymus reticulatus | Care | Reticulated Dragonet | Mollusc | Nucella lapillus | Nula | Dog Whelk |
| Mollusc | Calliostoma zizyphinum | Cazi | Painted Topshell | Crustacea | Pagurus bernardus | Pabe | Common Hermit Crab |
| Crustacea | Carcinus maenas | Cama | Shore Crab | Crustacea | Palaemon serratus | Pase | Common Prawn |
| Demersal | Centrolabrus exoletus | Ceex | Rock Cook | Mollusc | Pecten maximus | Pema | King Scallop |
| Demersal | Chelidonichthys lucerna | Chlu | Tub Gurnard | Demersal | Pollachius pollachius | Popo | Pollack |
| Demersal | Chirolophis ascanii | Chas | Yarrells Blenny | Demersal | Pomatoschistus microps | Pomi | Common Goby |
| Pelagic | Conger conger | Coco | Conger Eel | Demersal | Pomatoschistus minutus | Pomi | Sand Goby |
| Crustacea | Corystes cassivelaunus | Coca | Masked Crab | Demersal | Pomatoschistus pictus | Popi | Painted Goby |
| Demersal | Ctenolabrus rupestris | Ctru | Goldsinny | Demersal | Raja clavata | Racl | Thornback Ray |
| Mollusc | Crassostrea gigas | Crgi | Pacific oyster | Demersal | Raja undulata | Raun | Undulate Ray |
| Demersal | Dasyatis pastinaca | Dapa | Common Stingray | Pelagic | Scomber scombrus | Scsc | Mackerel |
| Demersal | Dicentrarchus labrax | Dila | Bass | Pelagic | Scyliorhinus canicula | Scca | Small Spotted Catshark |
| Crustacea | Diogenes pugilator | Dipu | Hermit Crab | Mollusc | Sepia officinalis | Seof | Common Cuttlefish |
| Mollusc | Doris pseudoargus | Dops | Sea Lemon | Demersal | Sparus aurata | Spau | Gilthead Sea Bream |
| Demersal | Eutrigla gurnadus | Eugu | Grey Gurnard | Demersal | Spondyliosoma cantharus | Spca | Black Seabream |
| Crustacea | Galathea spp. | Gasp | Squat Lobster | Mollusc | Steromphala cineraria | Stci | Grey Topshell |
| Pelagic | Galeorhinus galeus | Gaga | Tope Shark | Demersal | Symphodus melops | Syme | Corkwing Wrasse |
| Mollusc | Glycymeris glycymeris | Glgl | Dog Cockle | Demersal | Thorogobius ephippiatus | Thep | Leopard-Spotted Goby |
| Demersal | Gobiusculus flavescens | Gofl | Two-spot Goby | Demersal | Trisopterus luscus | Trlu | Bib |
| Demersal | Gobius paganellus | Gopa | Rock Goby | Demersal | Trisopterus minutus | Trmi | Poor Cod |
| Crustacea | Goneplax rhomboides | Gorh | Angular Crab | Mollusc | Tritia reticulata | Trre | Netted Dog Whelk |
| Crustacea | Hyas araneus | Hyar | Great Spider Crab | Mollusc | Trivia monacha | Trmo | European Cowries |
| Crustacea | Inachus dorsettensis | Indo | Scorpion Spider Crab | Mollusc | Trochidae | Trtr | Topshells |
| Demersal | Labrus bergylta | Labe | Ballan Wrasse | - | - | - | - |
Table A2. Species detected and the year(s) in which they were seen
| All | Just 2021 | Just 2022 | Just 2023 | Just 2024 | 2021 & 2022 | 2021 & 2023 | 2021 & 2024 | 2022 & 2023 | 2022 & 2024 | 2023 & 2024 |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
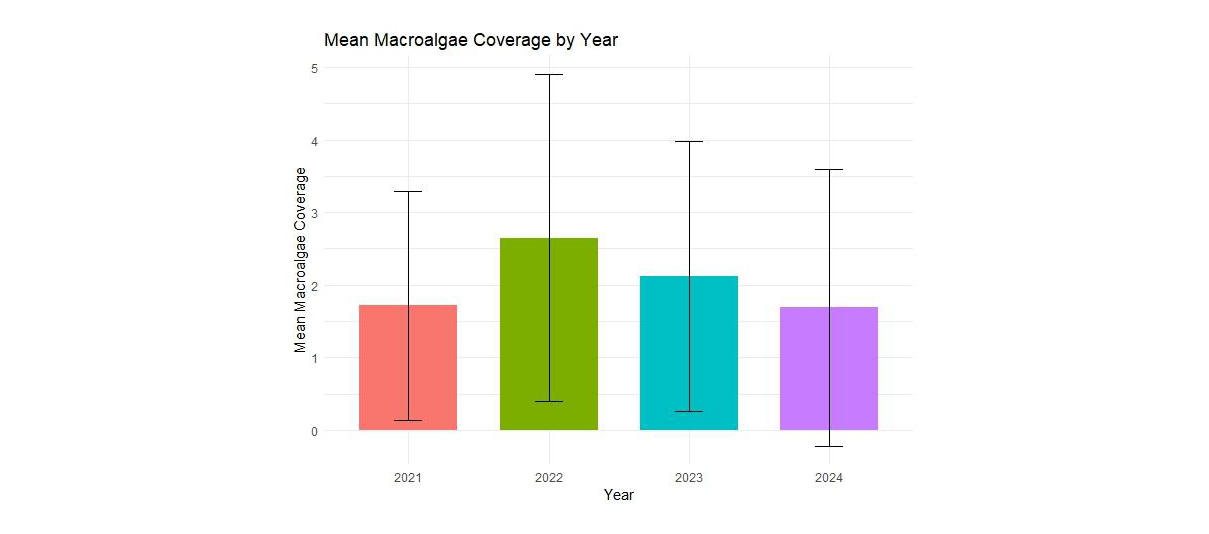 Figure A1. Mean macroalgal cover at 28 sites for each of the 4 years of sampling. A significant increase in average macroalgal cover was seen between 2021 and 2022 (GLM: Estimate= 0.43, Z-value= 2.34, p= 0.019). The macroalgal cover was not significantly different between other years.
Figure A1. Mean macroalgal cover at 28 sites for each of the 4 years of sampling. A significant increase in average macroalgal cover was seen between 2021 and 2022 (GLM: Estimate= 0.43, Z-value= 2.34, p= 0.019). The macroalgal cover was not significantly different between other years.
Report authors
- Alice Clark1
- Mika Peck1
- Valentina Scarponi1
- Emily Bulled2
- Ecology & Evolution, School of Life Sciences, University of Sussex, Falmer, Brighton BN1 9RH
- Blue Marine Foundation, Somerset House, London, WC2R 1LA
Citation
University of Sussex 2024. Monitoring recovery of habitats and species following the Sussex Nearshore Trawling Byelaw using Baited Remote Underwater Video. A report from surveys in 2021-2024 for Blue Marine Foundation.
© Blue Marine Foundation / University of Sussex 2024
This case study was adapted from a report published in December 2024: A collaborative study between Blue Marine Foundation and University of Sussex as part of the Sussex Kelp Recovery Project.
If you have any questions about the research, please contact Alice Clark (University of Sussex): c831@sussex.ac.uk
Acknowledgements
Thanks to Professor Mika Peck, Valentina Scarponi, Alice Clark, and Masters students at University of Sussex for undertaking surveys, data analysis, report editing and Francesco Marzano for collation of images and footage. Thanks also to Neville Blake, skipper of the New Dawn charter boat.
A final thank you to Prestige Flowers for sponsoring BRUV survey sites in partnership with GreenTheUK and the Blue Marine Foundation.
UN's Sustainable Development Goals
As a GreenTheUK partner, you support projects that are in line with the UN Sustainable Development Goals.

Take urgent action to combat climate change and its impacts.

Conserve and sustainably use the oceans, seas and marine resources for sustainable development.
















 mackerel shoal
mackerel shoal
 habitat
habitat
 mackerel shoal
mackerel shoal
 habitat
habitat
 habitat
habitat
 Atlantic Marckerel
Atlantic Marckerel
 Atlantic Marckerel
Atlantic Marckerel
 Habitat
Habitat